Breathtaking: New crystal steals oxygen from air, paves way for long, long plunge
Danish scientists have invented a revolutionary crystalline material that can absorb and store oxygen in high concentrations. A bucket of such material can suck the oxygen out of a room, which could potentially wave goodbye to heavy oxygen masks.
Imagine if you could get rid of the bulky scuba tank while taking a dive. Now, imagine practically any task to which the storage and timely release of oxygen is absolutely essential. And you have the new crystal developed at the University of Southern Denmark, with help from the University of Sydney, Australia.
A few microscopic grains are enough for one gulp of air, but a bucketful - or 10 liters - can completely suck the oxygen out of a room.
"In the lab, we saw how this material took up oxygen from the air around us,” Professor Christine McKenzie, who led the study, said.
When different things are exposed to oxygen, they react differently – from wine to food to living organisms, varying factors (pressure, temperature etc.) and time of exposure can fundamentally alter things. However, what you get with the new discovery is a way of controlling oxygen by not reacting with it.
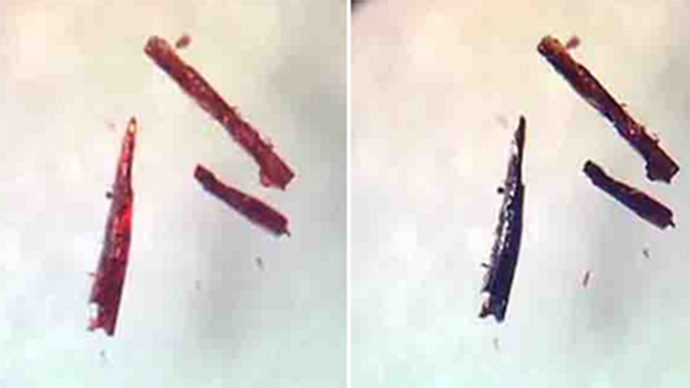
This was ascertained by using x-ray diffraction, showing the material’s atomic behavior when it was full of oxygen, then when it was depleted of it.
"An important aspect of this new material is that it does not react irreversibly with oxygen - even though it absorbs oxygen in a so-called selective chemisorptive process. The material is both a sensor, and a container for oxygen - we can use it to bind, store and transport oxygen – like a solid artificial hemoglobin," McKenzie says.
"It is also interesting that the material can absorb and release oxygen many times without losing the ability. It is like dipping a sponge in water, squeezing the water out of it and repeating the process over and over again," she continues.

To release the stored oxygen, all that is needed is to heat up the material, or pressure it. And those are just the known, natural ways. McKenzie and the team are now looking further than that.
“We are now wondering if light can also be used as a trigger for the material to release oxygen – this has prospects in the growing field of artificial photosynthesis," she says.
What may be surprising to some is how natural the process of storing the oxygen really is. The key component in the new material is the metal cobalt. It possesses a natural quality for absorption and “gives the new material precisely the molecular structure that enables it to absorb oxygen from its surroundings.”
There was also the question of time and speed: different conditions can affect this differently, and so can different versions of the material. This is crucial for the types of applications where controlled release is necessary – like in a gas mask or diving gear. Layering and arranging the material correctly will prevent its ability to just suck up the stuff from the surrounding air or water.
"When the material is saturated with oxygen, it can be compared to an oxygen tank containing pure oxygen under pressure - the difference is that this material can hold three times as much oxygen," McKenzie went on.
This could be valuable for lung patients who today must carry heavy oxygen tanks with them. But also divers may one day be able to leave the oxygen tanks at home and instead get oxygen from this material as it “filters” oxygen from its surroundings.














